 |
|
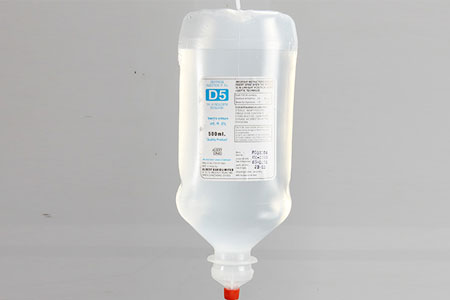
DEXTROSE 5% (P) IP 500 ml. |
|
Composition |
Each 100 ml contains |
Dextrose Anhydrous I.P : 5 gms
Water for Injection I.P. q.s. |
|
|
Therapeutic Area |
Supply of carbohydrate as energy source for
parenteral nutrition, prevention and treatment of
hypoglycemia and dilution of compatible drugs |
|
Dosage form |
Injectable |
|
Pack Size |
FFS Polyethylene Bottles: 500 ml. / Also available
in 1000 ml. |
|
|
 |
|
DEXTROSE 10% (G) 500ml |
|
Composition |
Each100 ml contains |
Dextrose Anhydrous I.P : 10 gms
Water for Injection I.P. q.s. |
|
|
Therapeutic Area |
Supply of carbohydrate as energy source for
parenteral nutrition, prevention and treatment of
hypoglycemia and dilution of compatible drugs |
|
Dosage form |
Injectable |
|
Pack Size |
U.S.P. Type I Glass Borosilicate Bottles 500 ml. |
|
|
 |
|
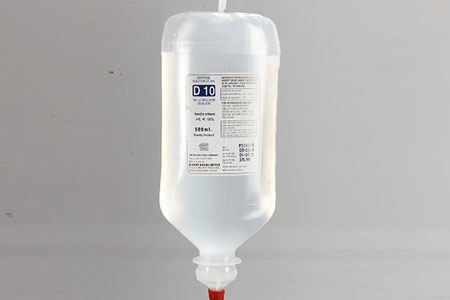
DEXTROSE 10% (P) IP 500ml. |
|
Composition |
Each100 ml contains |
Dextrose Anhydrous I.P : 10 gms
Water for Injection I.P. q.s. |
|
|
Therapeutic Area |
Supply of carbohydrate as energy source for
parenteral nutrition, prevention and treatment of
hypoglycemia and dilution of compatible drugs |
|
Dosage form |
Injectable |
|
Pack Size |
FFS Polyethylene Bottles : 500 ml. / Also available
in 1000 ml. pack |
|
|
 |
|
DEXTROSE 5% (G) IP 500ml. |
|
Composition |
Each100 ml contains |
Dextrose Anhydrous I.P : 5 gms
Water for Injection I.P. q.s. |
|
|
Therapeutic Area |
Supply of carbohydrate as energy source for
parenteral nutrition, prevention and treatment of
hypoglycemia and dilution of compatible drugs |
|
Dosage form |
Injectable |
|
Pack Size |
U.S.P. Type I Glass Borosilicate Bottles 500 ml. /
Also available in 1000 ml. pack |
|
|
 |
|
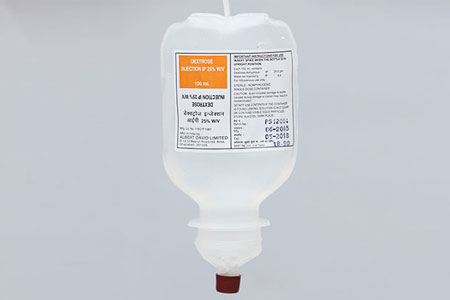
DEXTROSE INJ-25% IP 100ml. |
|
Composition |
Each100 ml contains |
Dextrose Anhydrous I.P :
25 gms
Water for Injection I.P. q.s. |
|
|
Therapeutic Area |
Supply of carbohydrate as energy source for
parenteral nutrition, prevention and treatment of
hypoglycemia and dilution of compatible drugs |
|
Dosage form |
Injectable |
|
Pack Size |
FFS Polyethylene Bottles : 100 ml. |
|
|
 |
|
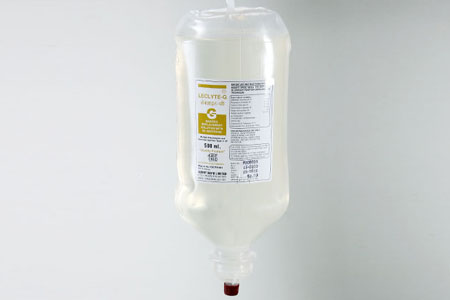
LECLYTE G (P) 500ml. |
|
Composition |
Each100 ml contains |
CDextrose Anhydrous I.P : 5.0 gms.
Potassium Chloride IP : 0.13 gms
Sodium Chloride IP : 0.37 gms
Ammonium Chloride IP : 0.37 gms
Added Sodium Sulphite IP : 15 mg
Water for Injection I.P. q.s.
Concentration of electrolytes (m.eq/Lt)
Na+ 63.0, K+ 17.0, NH4+ 70.0, Cl- 150.0 |
|
Therapeutic Area |
Replacement of abnormal gastric juice loss in
diarrhea, gastric suction,severe vomiting, metabolic
acidosis from excessive ingestion of Sodium
Bicarbonate. |
|
Dosage form |
Injectable |
|
Pack Size |
FFS Polyethylene Bottles: 500 ml. |
|
|
 |
|
LECLYTE M (P) 500ml |
|
Composition |
Each100 ml contains |
Dextrose Anhydrous I.P : 5.0 gms.
Potassium Chloride IP : 0.15 gms
Sodium Chloride IP : 0.10 gms
Sodium Acetate IP : 0.28 gms
Dipotassium Hydrogen Phosphate IP : 0.13 gms
Added Sodium meta-bi-sulphite 0.02% as anti-oxidant
Water for Injection I.P. q.s.
Concentration of electrolytes (m.eq/Lt)
Na+37.0, K+35.0, Cl-37.0, Acetate 20.0, PO4+7.5 |
|
Therapeutic Area |
Meet daily water and electrolyte requirements, mild
metabolic acidosis, water depletion due to reduced
water intake /excessive sweating |
|
Dosage form |
Injectable |
|
Pack Size |
FFS Polyethylene Bottles: 500 ml. |
|
|
 |
|
LECLYTE P (P) 500ml |
|
Composition |
Each100 ml contains |
Dextrose Anhydrous I.P : 5.0 gms.
Potassium Chloride IP : 0.13 gms
Sodium Acetate IP : 0.32 gms
Dipotassium Hydrogen Phosphate IP : 0.026 gms
Magnesium Chloride IP : 0.031 gms
Added Sodium meta-bi-sulphite 0.02% as anti-oxidant
Water for Injection I.P. q.s.
Concentration of electrolytes (m.mol/Lt)
Na+ 23.0, K+ 20.0, Mg+ 1.5, Cl- 20.0, Acetate 23.0,
PO4+ 1.5 |
|
Therapeutic Area |
Meet daily water and electrolyte requirements, mild
metabolic acidosis, water depletion due to reduced
water intake /excessive sweating |
|
Dosage form |
Injectable |
|
Pack Size |
FFS Polyethylene Bottles : 500 ml |
|
|
 |
|
LECLYTE G (G) 500ml |
|
Composition |
Each100 ml contains |
Dextrose Anhydrous I.P :
5.0 gms.
Potassium Chloride IP : 0.13 gms
Sodium Chloride IP : 0.37 gms
Ammonium Chloride IP : 0.37 gms
Added Sodium Sulphite IP : 15 mg
Water for Injection I.P. q.s.
Concentration of electrolytes (m.eq/Lt)
Na+ 63.0, K+ 17.0, NH4+ 70.0, Cl- 150.0 |
|
Therapeutic Area |
Replacement of abnormal gastric juice loss in
diarrhea, gastric suction,severe vomiting, metabolic
acidosis from excessive ingestion of Sodium
Bicarbonate. |
|
Dosage form |
Injectable |
|
Pack Size |
U.S.P. Type I Glass Borosilicate Bottles 500 ml. |
|
|
 |
|
LECLYTE - M (G) 500ml |
|
Composition |
Each100 ml contains |
Dextrose Anhydrous I.P : 5.0 gms.
Potassium Chloride IP : 0.15 gms
Sodium Chloride IP : 0.10 gms
Sodium Acetate IP : 0.28 gms
Dipotassium Hydrogen Phosphate IP : 0.13 gms
Added Sodium meta-bi-sulphite 0.02% as anti-oxidant
Water for Injection I.P. q.s.
Concentration of electrolytes (m.eq/Lt)
Na+37.0, K+35.0, Cl-37.0, Acetate 20.0, PO4+7.5 |
|
Therapeutic Area |
Meet daily water and electrolyte requirements, mild
metabolic acidosis, water depletion due to reduced
water intake /excessive sweating |
|
Dosage form |
Injectable |
|
Pack Size |
U.S.P. Type I Glass Borosilicate Bottles 500 ml. |
|
|
 |
|
LECLYTE P (G) 500ml |
|
Composition |
Each100 ml contains |
Dextrose Anhydrous I.P :
5.0 gms.
Potassium Chloride IP : 0.13 gms
Sodium Acetate IP : 0.32 gms
Dipotassium Hydrogen Phosphate IP : 0.026 gms
Magnesium Chloride IP : 0.031 gms
Added Sodium meta-bi-sulphite 0.02% as anti-oxidant
Water for Injection I.P. q.s.
Concentration of electrolytes (m.mol/Lt)
Na+ 23.0, K+ 20.0, Mg+ 1.5, Cl- 20.0, Acetate 23.0,
PO4+ 1.5 |
|
Therapeutic Area |
Meet daily water and electrolyte requirements, mild
metabolic acidosis, water depletion due to reduced
water intake /excessive sweating |
|
Dosage form |
Injectable |
|
Pack Size |
U.S.P. Type I Glass Borosilicate Bottles :500 ml |
|
|
 |
|
MANNITOL 20% (G) USP 350ml |
|
Composition |
Each100 ml contains |
Mannitol IP : 20 gms.
Water for Injection I.P. q.s. |
|
|
Therapeutic Area |
For reduction of intracranial pressure, brain volume
and intraocular pressure. Increase volume of urine
and sodium excretion |
|
Dosage form |
Injectable |
|
Pack Size |
U.S.P. Type I Glass Borosilicate Bottles 350 ml. |
|
|
 |
|
MANNITOL 20% (P) USP 100ml |
|
Composition |
Each100 ml contains |
Mannitol IP : 20 gms.
Water for Injection I.P. q.s. |
|
|
Therapeutic Area |
For reduction of intracranial pressure, brain volume
and intraocular pressure. Increase volume of urine
and sodium excretion |
|
Dosage form |
Injectable |
|
Pack Size |
FFS Polyethylene Bottles: 100 ml. |
|
|
 |
|
RINGER LACTATE (P) IP 500ml |
|
Composition |
Each100 ml contains |
Lactic Acid IP 0.24ml
equivalent to
Sodium Lactate : 0.32 gms.
Sodium Chloride IP : 0.6 gms
Potassium Chloride IP : 0.04 gms
Calcium Chloride IP : 0.027 gms
Water for Injection I.P. q.s.
Electrolyte concentration(approx.) mmol/Ltr
Na+131, K+5, Ca++ 2, bicarbonate (as lactate) 29 and
Cl-111 |
|
Therapeutic Area |
For restoration of normal fluid balance following
decreased intake or increased loss due to burns,
severe infections, peritonitis, cardiovascular
injuries. Also to combat moderate metabolic acidosis
following mild renal insufficiency, diarrhea in
infants, diabetic ketosis or excessive of acidifying
drugs (e.g. Salicylates). |
|
Dosage form |
Injectable |
|
Pack Size |
FFS Polyethylene Bottles
: 500 ml. |
|
|
 |
|
RINGER LACTATE (P) IP 1000ml |
|
Composition |
Each100 ml contains |
Lactic Acid IP 0.24ml equivalent to
Sodium Lactate : 0.32 gms.
Sodium Chloride IP : 0.6 gms
Potassium Chloride IP : 0.04 gms
Calcium Chloride IP : 0.027 gms
Water for Injection I.P. q.s.
Electrolyte concentration(approx.) mmol/Ltr
Na+ 131, K+5, Ca++ 2, bicarbonate (as lactate) 29
and Cl-111 |
|
Therapeutic Area |
For restoration of normal fluid balance following
decreased intake or increased loss due to burns,
severe infections, peritonitis, cardiovascular
injuries. Also to combat moderate metabolic acidosis
following mild renal insufficiency, diarrhea in
infants, diabetic ketosis or excessive of acidifying
drugs ( e.g. Salicylates). |
|
Dosage form |
Injectable |
|
Pack Size |
FFS Polyethylene Bottles : 1000 ml. |
|
|
 |
|
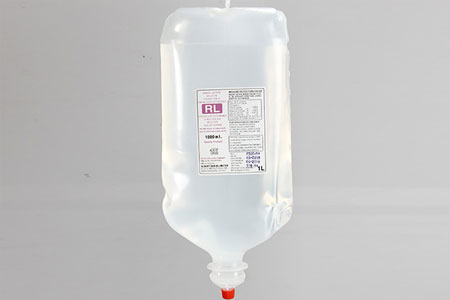
RINGER LACTATE (P) IP 1000ml |
|
Composition |
Each100 ml contains |
CLactic Acid IP 0.24ml equivalent to
Sodium Lactate : 0.32 gms.
Sodium Chloride IP : 0.6 gms
Potassium Chloride IP : 0.04 gms
Calcium Chloride IP : 0.027 gms
Water for Injection I.P. q.s.
Electrolyte concentration (approx.) mmol/Ltr
Na+ 131, K+5, Ca++ 2, bicarbonate (as lactate) 29
and Cl-111 |
|
Therapeutic Area |
For restoration of normal fluid balance following
decreased intake or increased loss due to burns,
severe infections, peritonitis, cardiovascular
injuries. Also to combat moderate metabolic acidosis
following mild renal insufficiency, diarrhea in
infants, diabetic ketosis or excessive of acidifying
drugs ( e.g. Salicylates). |
|
Dosage form |
Injectable |
|
Pack Size |
FFS Polyethylene Bottles : 1000 ml. |
|
|
 |
|
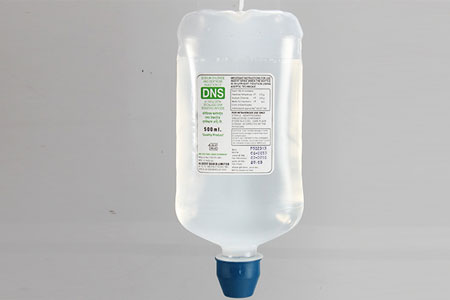
SODIUM CHLORIDE & DEXTROSE INJ. 500ml |
|
Composition |
Each100 ml contains |
Dextrose Anhydrous I.P : 5 gms
Sodium Chloride I.P : 0.9 gms
Water for Injection I.P. q.s.
Concentration of electrolytes millimols/Ltr (
approx.) :
Na+ : 150. Cl- : 150 |
|
Therapeutic Area |
As a source of calories, water and electrolytes
during parenteral nutrition , priming solution for
hemodialysis procedures etc. |
|
Dosage form |
Injectable |
|
Pack Size |
U.S.P. Type I Glass Borosilicate Bottles 500 ml. |
|
|
 |
|
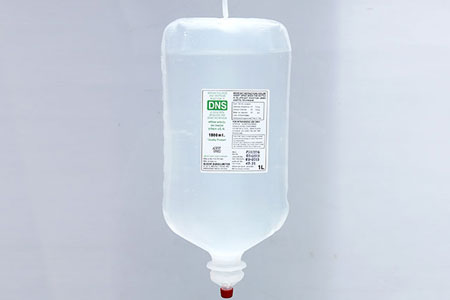
SODIUM CHLORIDE & DEXTROSE INJ. (P) 500ml |
|
Composition |
Each100 ml contains |
Dextrose Anhydrous I.P : 5 gms
Sodium Chloride I.P : 0.9 gms
Water for Injection I.P. q.s.
Concentration of electrolytes millimols/Ltr (
approx.) : Na+ : 150. Cl- : 150 |
|
Therapeutic Area |
As a source of calories, water and electrolytes
during parenteral nutrition , priming solution for
hemodialysis procedures etc. |
|
Dosage form |
Injectable |
|
Pack Size |
FFS Polyethylene Bottles : 500 ml. |
|
|
 |
|
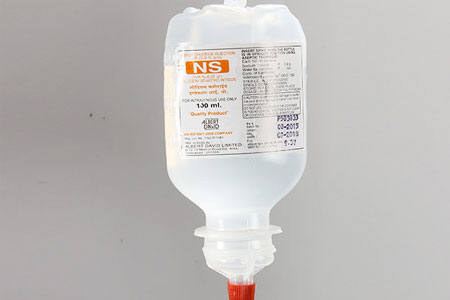
SODIUM CHLORIDE & DEXTROSE INJ. (P) 1000ml |
|
Composition |
Each100 ml contains |
Dextrose Anhydrous I.P : 5 gms
Sodium Chloride I.P : 0.9 gms
Water for Injection I.P. q.s.
Concentration of electrolytes millimols/Ltr (
approx.) : Na+ : 150. Cl-150 |
|
Therapeutic Area |
As a source of calories, water and electrolytes
during parenteral nutrition , priming solution for
hemodialysis procedures etc. |
|
Dosage form |
Injectable |
|
Pack Size |
FFS Polyethylene Bottles : 1000 ml. |
|
|
 |
|
SODIUM CHLORIDE INJ. (P) 100ml. |
|
Composition |
Each100 ml contains |
Sodium Chloride I.P : 0.9 gms
Water for Injection I.P. q.s.
Concentration of electrolytes millimols/Ltr (approx)
: Na+ : 150. Cl-150 |
|
Therapeutic Area |
As a source of water and electrolytes during
parenteral nutrition , priming solution for
hemodialysis procedures, excessive water and
electrolyte loss etc. |
|
Dosage form |
Injectable |
|
Pack Size |
FFS Polyethylene Bottles: 100 ml. |
|
|
 |
|
SODIUM CHLORIDE INJ. (P) 500ml. |
|
Composition |
Each100 ml contains |
Sodium Chloride I.P : 0.9 gms
Water for Injection I.P. q.s.
Concentration of electrolytes millimols/Ltr (approx)
: Na+ : 150. Cl-150 |
|
Therapeutic Area |
As a source of water and electrolytes during
parenteral nutrition , priming solution for
hemodialysis procedures, excessive water and
electrolyte loss etc. |
|
Dosage form |
Injectable |
|
Pack Size |
FFS Polyethylene Bottles: 500 ml. ml |
|
|
 |
|
SODIUM CHLORIDE INJ. (G) 500ml. |
|
Composition |
Each100 ml contains |
Sodium Chloride I.P : 0.9 gms
Water for Injection I.P. q.s.
Concentration of electrolytes millimols/Ltr (approx)
: Na+ : 150. Cl-150 |
|
Therapeutic Area |
As a source of water and electrolytes during
parenteral nutrition , priming solution for
hemodialysis procedures, excessive water and
electrolyte loss etc. |
|
Dosage form |
Injectable |
|
Pack Size |
U.S.P. Type I Glass Borosilicate Bottles 500 ml. /
Also available in 100 ml. pack |
|
|
 |
|
SODIUM CHLORIDE INJ. (P) 1000ml. |
|
Composition |
Each100 ml contains |
Sodium Chloride I.P : 0.9 gms
Water for Injection I.P. q.s.
Concentration of electrolytes millimols/Ltr (approx)
: Na+ : 150. Cl-150 |
|
Therapeutic Area |
As a source of water and electrolytes during
parenteral nutrition , priming solution for
hemodialysis procedures, excessive water and
electrolyte loss etc. |
|
Dosage form |
Injectable |
|
Pack Size |
FFS Polyethylene Bottles: 1000 ml. |
|
|
 |
|
SODIUM LACTATE INJ. (G) 500ml |
|
Composition |
Each100 ml contains |
Sodium Lactate I.P :
1.85 gms
Water for Injection I.P. q.s. |
|
Therapeutic Area |
Compound Sodium Lactate is used to replace body
fluid and mineral salts that may be lost for a
variety of medical reasons. It is especially
suitable when the losses result in too much acid
being present in the blood. |
|
Dosage form |
Injectable |
|
Pack Size |
U.S.P. Type I Glass Borosilicate Bottles 500 ml.
Also available in 500 ml. Polyethylene pack. |
|
|
|
|

























